Market Overview:
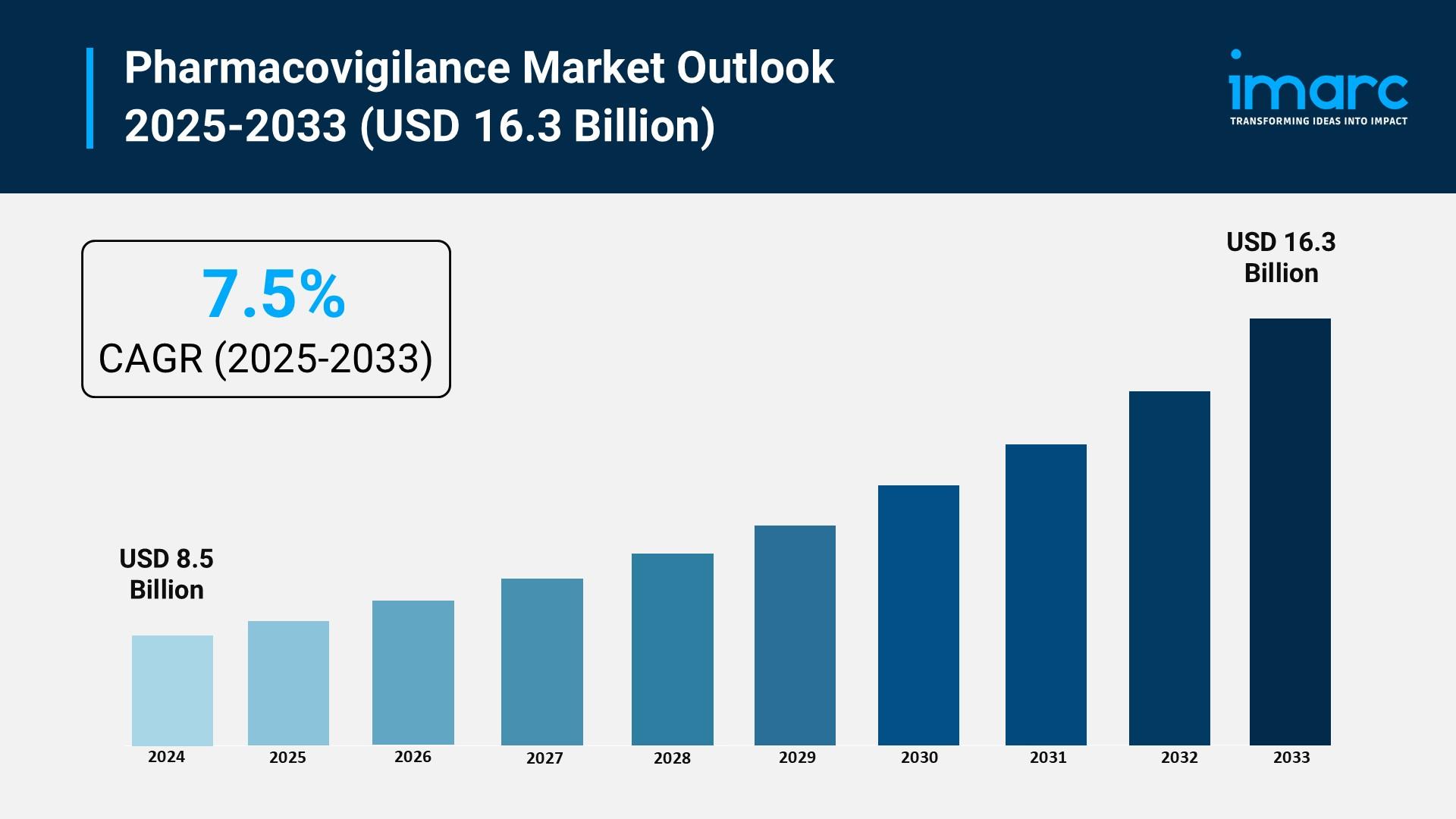
According to IMARC Group's latest research publication, "Pharmacovigilance Market: Global Industry Trends, Share, Size, Growth, Opportunity and Forecast 2025-2033", The global pharmacovigilance market size reached USD 8.5 Billion in 2024. Looking forward, IMARC Group expects the market to reach USD 16.3 Billion by 2033, exhibiting a growth rate (CAGR) of 7.5% during 2025-2033.
This detailed analysis primarily encompasses industry size, business trends, market share, key growth factors, and regional forecasts. The report offers a comprehensive overview and integrates research findings, market assessments, and data from different sources. It also includes pivotal market dynamics like drivers and challenges, while also highlighting growth opportunities, financial insights, technological improvements, emerging trends, and innovations. Besides this, the report provides regional market evaluation, along with a competitive landscape analysis.
How AI is Reshaping the Future of Pharmacovigilance Market
- AI and machine learning accelerate pharmacovigilance by reducing signal detection time by 40%, enhancing adverse event analysis through electronic health records and social media monitoring.
- European Medicines Agency released AI tools and guidelines in 2024 to improve drug safety monitoring, demonstrating regulatory support for technology adoption in pharmacovigilance operations.
- Sanofi and Deloitte's ConvergeHEALTH Safety platform uses AI to streamline case intake, while ArisGlobal's FAERS II integration enhances electronic safety submissions for faster regulatory reporting.
- Natural language processing algorithms process massive datasets from clinical trials and patient registries, improving signal detection accuracy and reducing manual workload by automating medical coding.
- AI-powered predictive analytics enable proactive risk management by anticipating adverse drug reactions, facilitating early interventions that minimize health complications and enhance patient safety across global markets.
Download a sample PDF of this report: https://www.imarcgroup.com/pharmacovigilance-market/requestsample
Key Trends in the Pharmacovigilance Market
- Surge in Contract Outsourcing Services: Pharmaceutical companies increasingly outsource pharmacovigilance to specialized CROs for cost efficiency and compliance. Contract outsourcing holds 61.2% market share, with 30% growth in agreements across North America and Europe in 2024, driven by access to advanced AI technologies.
- Real-World Evidence Integration: Patient registries and electronic health records revolutionize post-market surveillance, detecting rare adverse reactions missed in trials. European providers improved oncology drug monitoring in 2023, while UK pilot schemes increased patient-reported ADRs by 25% through mobile applications.
- Regulatory Framework Intensification: Global agencies like FDA, EMA, and CDSCO enforce stricter safety reporting standards. Aurobindo Pharma's nitrosamine contamination recall highlights rigorous testing requirements, driving pharmaceutical firms to reinforce internal systems and seek external expertise for compliance.
- Patient-Centric Reporting Methods: Digital platforms enable direct patient reporting of adverse drug reactions through mobile apps and online portals. Patient-reported outcomes improve detection accuracy and speed, with 2024 initiatives showing enhanced signal detection capabilities across decentralized clinical trial platforms.
- Decentralized Trial Monitoring: Adaptive protocols and patient-centric designs in clinical trials require agile safety systems. Over 22,000 new trials launched in 2023 feature decentralized elements, prompting Cognizant-Medable and Viedoc-LINK Medical partnerships for streamlined real-time monitoring across geographies.
Growth Factors in the Pharmacovigilance Market
- Rising Adverse Drug Reaction Incidents: Growing prevalence of ADRs from polypharmacy and substance abuse drives demand for robust monitoring systems. Chronic diseases like cancer, diabetes, and cardiovascular conditions increase simultaneous drug use, heightening interaction risks and safety surveillance needs.
- Expanding Drug Development Complexity: Novel biologics, gene therapies, and personalized medicine require specialized safety monitoring. The FDA cleared 50 new molecular entities in 2024, widening post-marketing safety duties with conditional approvals demanding continuous real-world evidence collection.
- Post-Marketing Surveillance Requirements: Phase IV studies dominate with 76.5% market share, mandated by regulatory agencies for long-term drug safety assessment. Higher ADR detection after approval necessitates continuous monitoring using big data analytics and real-world evidence integration.
- Technology Integration Acceleration: Machine learning and big data analytics enhance signal detection efficiency. The global ML industry reached USD 31 billion in 2024, with pharmacovigilance emerging as fastest-growing application, processing massive datasets at unprecedented speed and scale.
- Outsourcing Trend Expansion: Pharmaceutical firms focus on core drug discovery by delegating safety monitoring to expert CRO partners. Specialized providers offer scalable infrastructure, experienced analysts, and regional regulatory intelligence, enabling compliant global market coverage while reducing operational costs.
Leading Companies Operating in the Global Pharmacovigilance Industry:
- Accenture plc
- ArisGlobal LLC
- BioClinica Inc. (Cinven Partners LLP)
- Capgemini
- Cognizant
- International Business Machines Corporation
- ICON plc
- IQVIA Inc.
- ITClinical
- Parexel International Corporation
- Wipro Limited
Pharmacovigilance Market Report Segmentation:
Breakup By Service Provider:
- In-house
- Contract Outsourcing
Contract outsourcing accounts for the majority of shares (61.2%) due to cost efficiency, access to specialized expertise, and regulatory compliance capabilities.
Breakup By Product Life Cycle:
- Pre-clinical
- Phase I
- Phase II
- Phase III
- Phase IV
Phase IV dominates with 76.5% market share owing to mandatory post-marketing surveillance and long-term drug safety evaluation requirements.
Breakup By Type:
- Spontaneous Reporting
- Intensified ADR Reporting
- Targeted Spontaneous Reporting
- Cohort Event Monitoring
- EHR Mining
Spontaneous reporting leads with 31.9% share due to its widespread usage in identifying adverse drug reactions cost-effectively and efficiently.
Breakup By Process Flow:
- Case Data Management
- Signal Detection
- Risk Management System
- Fully Integrated Software
Signal detection holds the largest segment (38.2%) as it plays a central role in detecting and managing safety concerns throughout drug development and post-launch phases.
Breakup By Therapeutic Area:
- Oncology
- Neurology
- Cardiology
- Respiratory Systems
- Others
Oncology accounts for the majority of shares (27.8%) due to high numbers of clinical trials, approved drugs, and increasing global cancer prevalence driving comprehensive safety measures.
Breakup By End Use:
- Pharmaceutical Companies
- Biotechnology Companies
- Medical Device Companies
- Others
Pharmaceutical companies dominate with 44.2% share because of significant responsibilities in drug development, safety monitoring, and meeting regulatory compliance requirements.
Breakup By Region:
- North America (United States, Canada)
- Asia Pacific (China, Japan, India, South Korea, Australia, Indonesia, Others)
- Europe (Germany, France, United Kingdom, Italy, Spain, Russia, Others)
- Latin America (Brazil, Mexico, Others)
- Middle East and Africa
North America enjoys the leading position (33.7% share) owing to advanced healthcare infrastructure, stringent FDA regulations, and widespread adoption of AI and big data for safety monitoring.
Recent News and Developments in Pharmacovigilance Market
- January 2025: Clinigen formed a strategic partnership with Tepsivo, acquiring a minority stake in the global digital pharmacovigilance services provider, combining expertise in PV, regulatory affairs, and medical information with innovative technology to modernize services.
- August 2024: The Pharmacovigilance Programme of India (PvPI) launched ADRMS software as part of the 'Digital India' initiative, creating India's first safety database for adverse event reporting by patients, doctors, nurses, and pharmaceutical industries.
- June 2024: Accenture announced a $3 billion investment into its Data & AI practice, aiming to double AI talent and develop new AI-driven solutions, including enhancements in pharmacovigilance for automated adverse event detection.
- May 2024: GSK acquired Affinivax, Inc. for $2.1 billion upfront and up to $1.2 billion in development milestones, gaining access to a novel 24-valent pneumococcal vaccine candidate to enhance vaccine technology portfolio.
- January 2024: Cognizant was selected as strategic technology transformation provider for Fortrea, deploying a modern digital ecosystem with hybrid cloud and next-generation platforms to accelerate treatment delivery to patients globally.
Note: If you require specific details, data, or insights that are not currently included in the scope of this report, we are happy to accommodate your request. As part of our customization service, we will gather and provide the additional information you need, tailored to your specific requirements. Please let us know your exact needs, and we will ensure the report is updated accordingly to meet your expectations.
About Us:
IMARC Group is a global management consulting firm that helps the world's most ambitious changemakers to create a lasting impact. The company provide a comprehensive suite of market entry and expansion services. IMARC offerings include thorough market assessment, feasibility studies, company incorporation assistance, factory setup support, regulatory approvals and licensing navigation, branding, marketing and sales strategies, competitive landscape and benchmarking analyses, pricing and cost research, and procurement research.
Contact Us:
IMARC Group
134 N 4th St. Brooklyn, NY 11249, USA
Email: sales@imarcgroup.com
Tel No:(D) +91 120 433 0800
United States: +1-201971-6302